Prescription Required

Authentic Product

India's Leading Generic Pharmacy

Secure Payment
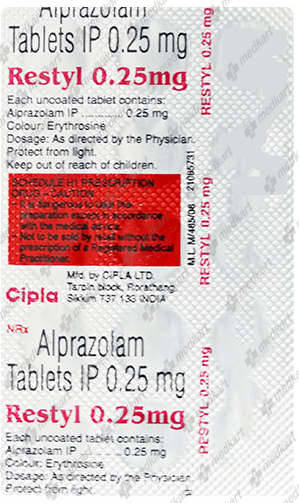
RESTYL 0.25MG TABLET 15'S
By CIPLA PHARMACEUTICAL COMPANY LIMITED
MRP
₹
34.06
₹28.95
15 % OFF
₹1.93 Only /
TabletCheck Delivery
--
Composition
Product Details
About RESTYL 0.25MG TABLET 15'S
RESTYL 0.25MG TABLET 15'S is a prescription medication that belongs to the class of drugs known as benzodiazepines. It is commonly prescribed to treat anxiety disorders, panic disorders, and anxiety associated with depression. RESTYL 0.25MG TABLET 15'S works by enhancing the effects of a neurotransmitter called gamma-aminobutyric acid (GABA) in the brain, which helps to calm the excessive activity in the central nervous system. It is available in tablet form and is typically taken orally.,The use of RESTYL 0.25MG TABLET 15'S should be closely monitored, as it has the potential to be habit-forming. It is important to take this medication exactly as prescribed by a healthcare professional and to follow the recommended dosage and duration of treatment. Abruptly stopping RESTYL 0.25MG TABLET 15'S can lead to withdrawal symptoms, such as rebound anxiety, insomnia, irritability, and seizures. Therefore, it is generally recommended to gradually reduce the dosage under medical supervision when discontinuing the medication.,While RESTYL 0.25MG TABLET 15'S can be effective in managing anxiety and panic disorders, it does come with some potential side effects. Common side effects may include drowsiness, dizziness, impaired coordination, and memory problems. Some individuals may also experience paradoxical reactions, such as increased anxiety or aggression. It is important to discuss any concerns or side effects with a healthcare provider to ensure the safe and appropriate use of RESTYL 0.25MG TABLET 15'S.
Dosage Details
- For Children:
- RESTYL 0.25MG TABLET 15'S is generally not recommended for use in children under the age of 18, as its safety and efficacy in pediatric populations have not been well-established. Alternative medications or treatments may be considered for children with anxiety or panic disorders.
- For Adults:
- Anxiety Disorders: The initial dosage typically ranges from 0.25 mg to 0.5 mg taken three times per day. The dosage may be adjusted gradually based on the individual's response and tolerance, up to a maximum daily dose of 4 mg.
- Panic Disorder: The starting dosage is often 0.5 mg taken three times per day. The dosage can be increased incrementally, as needed, up to a maximum daily dose of 10 mg.
Side Effects of RESTYL 0.25MG TABLET 15'S
- Drowsiness
- Fatigue
- Dizziness
- Cognitive Impairment
- Slurred Speech
- Confusion
- Gastrointestinal Issues
- Dry Mouth
- Changes in Libido
- Withdrawal Symptoms
Storage Information
- Store RESTYL 0.25MG TABLET 15'S at room temperature (68°F to 77°F or 20°C to 25°C).
- Keep RESTYL 0.25MG TABLET 15'S away from extreme heat or cold.
- Protect the medication from moisture by storing it in a dry place.
- Avoid storing RESTYL 0.25MG TABLET 15'S in the bathroom where humidity levels are higher.
FAQs
What is RESTYL 0.25MG TABLET 15'S used for?

RESTYL 0.25MG TABLET 15'S is used primarily for the treatment of anxiety disorders, panic disorders, and anxiety associated with depression.
How does RESTYL 0.25MG TABLET 15'S work?

RESTYL 0.25MG TABLET 15'S works by enhancing the activity of the neurotransmitter GABA, which has calming and sedative effects in the brain.
Is RESTYL 0.25MG TABLET 15'S addictive?

Yes, RESTYL 0.25MG TABLET 15'S has the potential for dependence and addiction, especially with long-term use or misuse.
What are the common side effects of RESTYL 0.25MG TABLET 15'S ?

Common side effects of RESTYL 0.25MG TABLET 15'S include drowsiness, fatigue, dizziness, cognitive impairment, slurred speech, confusion, gastrointestinal issues, dry mouth, changes in libido, and withdrawal symptoms if abruptly stopped.
Can RESTYL 0.25MG TABLET 15'S be used in children?

RESTYL 0.25MG TABLET 15'S is generally not recommended for use in children under the age of 18, as its safety and efficacy in pediatric populations have not been well-established.
How long can RESTYL 0.25MG TABLET 15'S be taken?

RESTYL 0.25MG TABLET 15'S is typically prescribed for short-term use due to its potential for dependence and addiction. The duration of treatment depends on the individual's specific condition and treatment plan.
Can RESTYL 0.25MG TABLET 15'S be taken during pregnancy or breastfeeding?

It is generally not recommended to take RESTYL 0.25MG TABLET 15'S during pregnancy or while breastfeeding, as it may pose risks to the developing fetus or newborn. Pregnant or breastfeeding individuals should consult their healthcare provider for guidance.
Can RESTYL 0.25MG TABLET 15'S interact with other medications?

Yes, RESTYL 0.25MG TABLET 15'S can interact with other medications, including opioids, alcohol, certain antidepressants, and other central nervous system depressants. These interactions can increase the risk of sedation, respiratory depression, and other adverse effects. It is important to inform your healthcare provider about all medications and substances you are taking to avoid potential interactions.
Ratings & Review
Best place for generic medicine at the cheapest rate
PATHAN HUNAIDKHAN
•
Reviewed on 03-04-2022
(5/5)
Visited medkart and it was a great experience with staff and they explains everything about generic medicine. How they r less in cost and both continent and power are same., everyone who buy their monthly medicine from other medicine stores should visit medkart and convert to generic medicine........ Best of luck medkart
Solanki Girish
•
Reviewed on 19-04-2023
(5/5)
I recently had a great experience with Medkart Pharmacy and wanted to share my positive review. The service they provided for medicines was outstanding. The staff was not only knowledgeable but also very friendly and eager to assist. They helped me find the right medicines and even provided valuable information about potential interactions and side effects. Furthermore, the pharmacy had a wide range of medicines in stock, making it convenient for me to get everything I needed in one place. Their prices were competitive, and I appreciated the affordability of the medications. I also want to highlight their quick and efficient service. My prescriptions were filled promptly, and I didn't have to wait long at all. Overall, I highly recommend Medkart Pharmacy for their excellent service, knowledgeable staff, and convenience. They made the process of getting my medicines easy and stress-free. I'll definitely be returning for my future pharmaceutical needs.
Harendra Kumawat
•
Reviewed on 14-09-2023
(5/5)
Good
tarif Malek
•
Reviewed on 15-01-2024
(5/5)
Good place to buy generic medicines
Patel Jinal
•
Reviewed on 24-05-2023
(5/5)
Marketer / Manufacturer Details
TETCIPLA PHARMACEUTICAL COMPANY LIMITED
Country of Origin -
India
Alternatives
Customer Also Bought
Related Blogs
Disclaimer
Medkart's sole intention is to ensure that its consumers get information that is expert-reviewed, accurate and trustworthy. However, the information contained herein should NOT be used as a substitute for the advice of a qualified physician. The information provided here is for informational purposes only. This may not cover everything about particular health conditions, medicines, generic alternatives, all possible side effects, drug interactions, warnings, alerts, lab tests, etc. Please consult your doctor and discuss all your queries related to any disease or medicine. We intend to support, not replace, the doctor-patient relationship.
India's most trusted generic medicine pharmacy.
10 Lakh+
Happy customers
35000+
Pin-codes Covered
75 Lakh+
Orders Delivered
Authentic Products
All WHO-GMP Certified Medicines
Download the app for free
©2025 Medkart Pharmacy. All Rights Reserved